Mohammed Haneefa Nizamudeen
Pursue what captures your heart, not what captures your eyes“– Roy T. Bennett
Today, we put Vigor Rehabs ( NASDAQ: VERV) in the spotlight. Vigor intends to change treatment through in vivo gene treatment utilizing base modifying innovation, with its lead prospect VERVE-101 revealing appealing lead to lowering LDL-C levels. An analysis follows listed below.
Business Summary
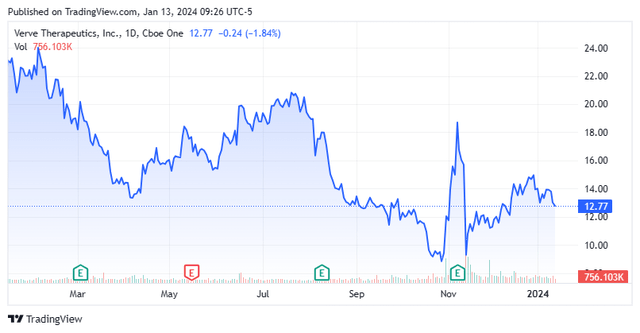
Vigor Rehabs, Inc. is a Boston based early clinical-stage hereditary medications issue concentrated on the advancement of single-course modifying techniques to deal with heart disease[CVD] The business as one program in the center and 2 more going through IND-enabling research studies. Vigor began operations in 2018 and went public in June 2021, raising net profits of $281.6 million at $19 per share. After a hot IPO – that opened at $30 and reached $73.80 9 trading sessions later on – its stock trades simply under $14.00 a share, equating to a market cap of $1.05 billion.
Heart Disease
The business’s main target in its effort to minimize CVD is low density lipoprotein-cholesterol (LDL-C), a substance whose assistance is greatly affected by binding to the PCSK9 protein. LDL-C is thought to promote atherosclerotic plaque that can advance to solidified arteries, the main quality of atherosclerotic CVD (ASCVD), which manifests as coronary heart problem, ischemic stroke, and peripheral vascular illness. This thought connection is strengthened by research studies revealing a lowering of LDL-C by 39mg/dL for 5 years with recognized ASCVD minimizes the threat of another occasion by 21%. That very same LDL-C decrease over the life time of a private, cuts the threat of a very first ASCVD occasion by 88%.
The present paradigm for reducing LDL-C includes (primarily) everyday intake of statin drugs, which according to Dr. Perlmutter in his book Grain Brain, ups the threat for Alzheimer’s illness by 50%. 2 monoclonal antibodies developed to bind to the PSCK9 protein have actually been authorized – Amgen’s ( AMGN) Repatha (evolocumab) and Regeneron’s ( REGN) Praluent (alirocumab) – however they need two times month-to-month injections. Likewise, Novartis’ ( NVS) Leqvio (inclisiran) is a two times annual little interfering RNA subcutaneous injection that similarly targets PCSK9. Throughout 3 research studies, Leqvio showed an LDL-C decrease of 47.4% at day 510. Blunt force workarounds consist of the insertion of stents and coronary bypass. In spite of these ‘improvements’ in care, 4 out of 5 clients do not accomplish their LDL-C objectives and CVD stays the leading cause of death worldwide, with roughly one in 3 catching the ailment. Moreover, according to the CDC, CVD saps over $350 billion every year out of American wallets (over $1,000 per person) in health care expenses and lost performance.
Vigor’s Service( s)
Vigor intends to turn the present treatment paradigm on its head through single administration ( in vivo) gene treatment that utilizes base modifying innovation. To even more show the business’s method, the presently en vouge CRISPR-Cas gene modifying innovation includes splicing the double-stranded DNA with the ensuing self-repair developed to knockout the gene. By contrast, Vigor’s base modifying method belongs to a pencil that removes and rewords one letter in the gene. This is achieved through a substance including a lipid nanoparticle that encapsulates both the mRNA encoding for the gene (or base) editor and a guide RNA targeting the gene of interest. In theory, this more customized and accurate method ought to cause less undesirable DNA adjustments or off-target modifying results. Although in its early phase, Vigor is the only business advancing an in vivo CVD gene treatment in the center, offering it prospective first-mover benefit.
VERVE-101
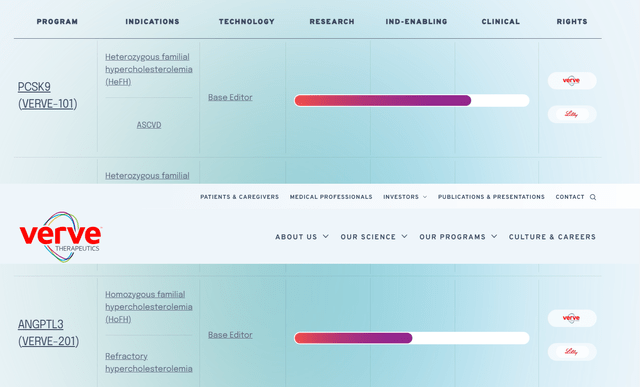
That prospect, VERVE-101, is developed to completely disable the PCSK9 gene in the liver in order to knockout the production of PCSK9 protein. Through its decrease, the liver can more quickly clear LDL-C from the blood. VERVE-101 is going through assessment for the treatment of heterozygous familial hypercholesterolemia (HeFH), a congenital disease defined by LDL-C levels in between 200 and 400mg/dL (normal/good: 70 to 130mg/dL) that affects ~ 1.3 million Americans. This is not to be puzzled with homozygous familial hypercholesterolemia (HoFH), which is defined by 2 mutant alleles of the LDLR gene – HeFH has actually one altered allele – and LDL-C levels north of 500mg/dL that affects ~ 1,300 Americans.
In the preclinic, a single one to 2 hour infusion of VERVE-101 was revealed to minimize LDL-C levels by 50% (0.75 mg/kg) and 68% (1.5 mg/kg) in non-human primates at 1 year post-treatment, which set that phase for a Stage 1b medical trial (heart-1) that dosed its very first of ~ 40 HeFH clients with recognized ASCVD in July 2022. Nevertheless, the trial was just provided a thumbs-up in New Zealand and the UK, as the FDA positioned a hang on Vigor’s IND in December 2022, asking for different details, consisting of offered medical information from heart-1, along with a demand to customize its trial procedure in the U.S. to (among other procedures) increase the length of the shocking period in between dosing of individuals.
The hold was lastly raised in October 2023 with very first information from New Zealand and the UK launched on November 12, 2023. The bright side: the effectiveness information was favorable with the evaluable clients treated with a healing level of VERVE-101 (0.45 mg/kg n= 2; 0.60 mg/kg n= 1) attaining LDL-C decreases of 39%, 48%, and 55% (0.60 mg/kg client) a minimum of one month after infusion.
Nevertheless, one client in the low dosage mate (0.30 mg/kg n= 6) suffered a deadly cardiovascular disease (considered not treatment associated) while one client in the 0.45 mg/kg mate experienced a Grade 3 myocardial infarction that was considered possibly treatment associated due to the distance to dosing (one day post-treatment). It ought to be kept in mind that the client had unsteady chest discomfort signs prior to dosing that went unreported to detectives. That very same client likewise suffered an unassociated Grade 2 non-sustained ventricular tachycardia (fast heart beat) 4 weeks post-treatment. This news sent out shares of VERV into a one-day 41% decrease to $9.29 a share, instant squashing possibly favorable news concerning its partnership with Beam Rehabs ( BEAM) revealed back on October 31, 2023. More on that advancement soon.
Pre-Clinical Possessions
In addition to VERVE-101, the business has comparable working possessions in the preclinic. One, VERVE-102, is basically VERVE-101 with a customized shipment lorry that consists of a N-Acetylgalactosamine lipo-nanoparticle that allows intro through either the LDLR receptor (like VERVE-101) or ASGPR hepatocyte receptor to lower LDL-C. Initiation of a Stage 1b research study is anticipated in 1Q24.
Vigor’s other possession going through IND-enabling research studies is VERVE-201, an ANGPTL3 targeting gene treatment for the treatment of HoFH that ought to get in the center in 2H24.
Cooperation News
The base editor innovation for VERVE-101, -102, and -201 was in-licensed from Beam under a 2019 arrangement that was modified in 2022. For the PCSK9 and ANGPTL3 gene treatments, after the last client has actually been dosed in a Stage 1, Beam deserves to opt-in to each program, which makes up covering 33% of the medical expenditures and a 50/50 share of revenues in the U.S. An opt-in to a 3rd gene target by Beam would obligate it to 35% of the advancement expenditures and 35% of the earnings share worldwide.
That plan was rather customized when Eli Lilly participated in a contract with Beam to get particular item rights to all 3 Vigor targets for a factor to consider of $200 million in advance, a $50 million equity financial investment in Beam, and prospective turning point commitments amounting to $350 million.
That news begins the heels of Vigor’s own arrangement with Lilly in June 2023, under which the latter gotten a license from the previous to establish a lipoprotein( a) targeted gene treatment for the treatment of ASCVD for a factor to consider of $30 million in advance, a $30 million stock financial investment, prospective turning points of $465 million, and high single-digit to low double-digit royalties. Vigor has the choice to bypass the turning points and royalties by opting-in after Stage 1 for a concealed cost in return for a 40% of the earnings share along with 40% of the advancement expenditures after Stage 1.
Vigor likewise has a 2022 partnership arrangement with Vertex Pharmaceuticals ( VRTX), under which it got an overall preliminary factor to consider of $60 million ($ 25 million money; $35 million equity financial investment) for one target. Vertex is possibly on the hook for turning points amounting to $406 million and single digit royalties.
Balance Sheet & & Expert Commentary
Although the marketplace responded adversely to the early information on VERVE-101, the business carried out secondary offerings on November 28, 2023, raising overall net profits of $157.7 million at $10 per share, consisting of a personal positioning acquired by Lilly that represented $23.0 countless the overall funds raised. That ought to put money and financial investments someplace in the area of $610 million with one month staying in 2023, supplying a money runway into 2026.
In spite of the marketplace’s uninterested response to VERVE-101 information, Street experts lean favorable on Vigor. Because 3rd quarter numbers were published in early November, 4 expert companies consisting of RBC Capital and BMO Capital have actually reissued Buy rankings on the stock. Rate targets proffered variety from $32 to $56 a share. JP Morgan preserved its Hold score and $27 cost target.
GV 2023 GP, LLC, represented on the board by Krishna Yeshwant, acquired 1.8 million shares on the secondary, raising its ownership interest to 15%.
Decision:
Internet of balance sheet money, the marketplace worths Vigor’s one-off, in vivo eraser-and-pencil method at ~$ 450 million. There is little in the method of information, however there is likewise little in the method of competitors for a one-and-done method. Just CRISPR Rehabs ( CRSP) has a one-off in vivo possession on the drawing board (CTX330) targeting CVD which is still in the discovery stage. Although extremely early in the video game, if additional security information on VERVE-101 can confirm that the negative occasion in the 0.45 mg/kg client was a fluke – implying greater dosage levels are possible – Vigor might be off to the races, as the effectiveness element of its method does not seem much in concern.
Undoubtedly, this is a coin turn. Nevertheless, a ‘ heads‘ result not just produces a considerable increase in its share cost – shares of VERV traded at $78 soon after the business’s IPO in September 2021 – however likewise presents the probability of a buyout by Eli Lilly, who is energetically browsing a significant stake in the CVD gene treatment arena. A ‘ tails‘ result leaves the business with (presently) ~$ 7.50 a share in money and 2 more possessions going into the center in 2024. As such, for the threat tolerant, Vigor deserves a little financial investment pending additional advancements.
When the heart speaks, the mind discovers it indecent to object.“– Milan Kundera